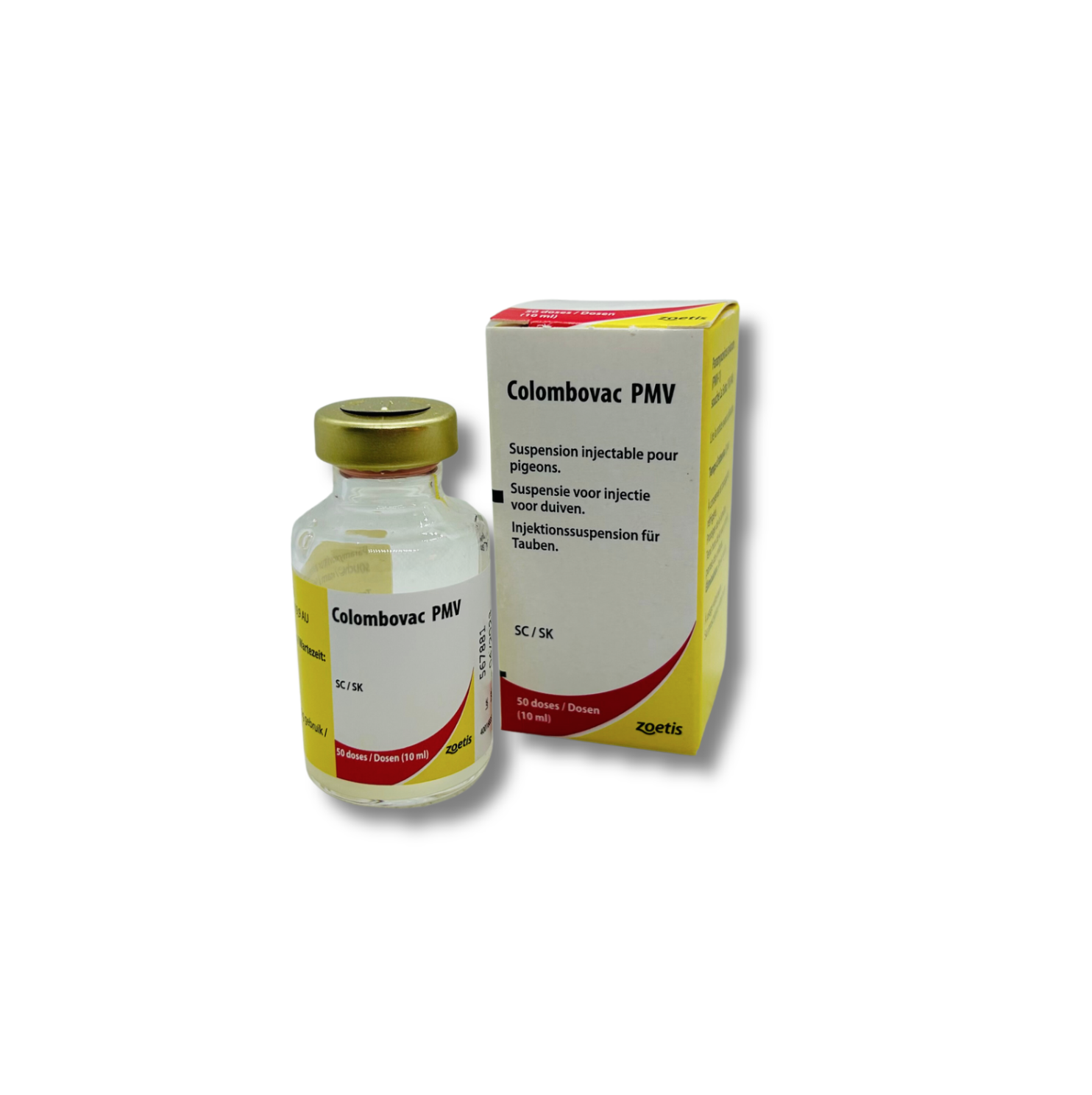
Zoetis Colombovac PMV / POX Paramixo – 50ds
€ 38,50
For the active immunisation of pigeons to prevent mortality and clinical signs due to paramyxo virus type 1 infection. The onset of protection occurs one month after inoculation, the duration of protection is 12 months.
Out of stock
Description
Zoetis Colombovac PMV/POX Paramixo virus for pigeons (50ds)
Indication
For active immunisation from the age of 6 weeks against diseases caused by pigeons paramyxovirus type 1 and pigeon pox virus.
Content of active and other ingredients
Per dose of 0.2 ml:
Active ingredients: Inactivated Newcastle disease virus, strain LaSota; at least 19.9 AU (AU: Antigen Unit).
Lyophilised fraction: Freeze-dried live pigeonpea virus, strain DD, at least 103.5 CCID50 (Cell Culture Infective Dose 50%)
Liquid fraction:
Adjuvants: Carbomer 934P (carbopol) 1 mg
Excipients: Thiomersal 20 μg
Dosage
0.2 ml per animal, administered subcutaneously only.
Method of use
Vaccination schedule:
– Basic vaccination: single vaccination, with one dose per animal from 6 weeks of age
– Repeat vaccination: annual single vaccination with one dose per animal.
Instructions for correct administration
The liquid Colombovac PMV fraction is intended as a suspending solution for the freeze-dried Colombovac POX fraction.
Side effects
At the injection site, transient swelling up to about 1 cm in diameter may be very common; it may persist for up to 4 weeks or longer. The swelling normally disappears without treatment. In cases where the side effects do not disappear spontaneously, the veterinarian should be contacted.
If you notice serious side effects or other types of reactions not mentioned in this leaflet, you are requested to inform your veterinarian.
Waiting period
Zero days.
Special precautions during storage
– Store at 2°C-8°C (in a refrigerator).
– Protect from light.
– Shelf life after first opening vial: use immediately, do not store.
– Do not use after the expiry date stated on the label.
– Keep out of the sight and reach of children.
Special warnings
Special precautions for use in animals:
Intramuscular administration of the product should be avoided.
Special warnings for each animal species for which the veterinary medicinal product is intended: Maternal antibodies may adversely affect the result of vaccination.
Special precautions to be taken by the person administering the veterinary medicinal product to the animals: In case of accidental self-injection, a doctor should be consulted immediately.
| Classificatie | UDD |
|---|


 Pharmagal Bio Colvac RP - Rotavirus. Paramyxovirus.
Pharmagal Bio Colvac RP - Rotavirus. Paramyxovirus.